MENU
FR | EUR
-
-
-
-
- Services pour bioprocédés
- Services pour centrifugeuse et rotors
- Service pour pipette
- Services pour Mastercycler
- Services pour automates de pipetage
- Services pour congélateurs
- Services pour incubateurs
- Services pour agitateurs
- Services pour appareils de photométrie
- Service de contrôle de la température et de l’agitation
-
-
-
-
-
- Services pour bioprocédés
- Services pour centrifugeuse et rotors
- Service pour pipette
- Services pour Mastercycler
- Services pour automates de pipetage
- Services pour congélateurs
- Services pour incubateurs
- Services pour agitateurs
- Services pour appareils de photométrie
- Service de contrôle de la température et de l’agitation
-
FR | EUR
-
- Centrifugeuses de paillasse
- Centrifugeuses au sol
- Centrifugeuses réfrigérées
- Microcentrifugeuses
- Centrifugeuses multi-fonctions
- Centrifugeuses haute vitesse
- Ultracentrifugeuses
- Concentrateur
- High-Speed and Ultracentrifugation Consumables
- Accessoires
- Tubes
- Plaques
- Gestion des appareils
- Gestion des échantillons et des informations
- Produits IVD
Aucun résultat trouvé
Chercher des suggestions

It’s a Matter of Volume: Bioprocess Scalability
Lab Academy
- Bioprocédés
- Culture cellulaire
- Reproductibilité
- Modularité
- Bioprocédés
- Test
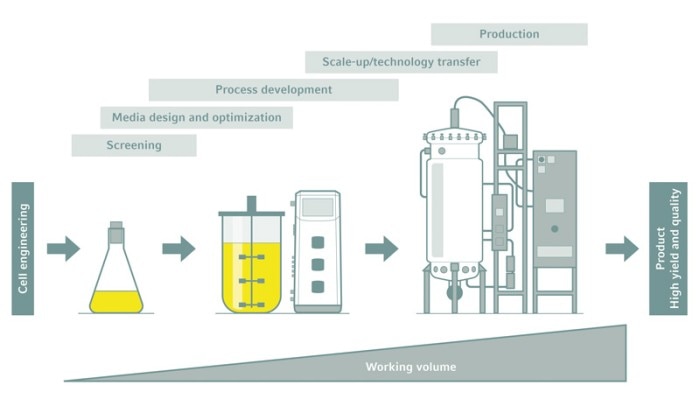
Regardless what kind of production process a bioreactor or fermentor is used for, efficiencies of time and cost are major factors to consider. Biopharmaceuticals, enzymes, biological cell materials or food supplements, for example, are all derived from the cultivation of bacteria, fungi or animal cells in bioreactors. These bioprocesses are usually developed at small laboratory scale. Later, the established processes are stepwise transferred to larger volumes until the final industrial production-scale is reached. This procedure is known as scale-up.
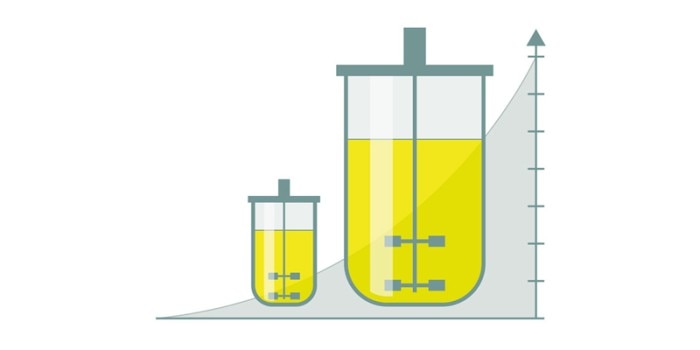
Scalability—an important aspect in bioprocessing
Several factors play a role in a reliable bioprocess scale-up [1, 2]. Kinetics and thermodynamics are virtually unaffected by the reaction volume. However, the mass transfer within a process is highly dependent on the scale. The geometries of the bioreactor and the impeller influence the mixing time and as such the oxygen uptake, substrate supply of a culture and the reagents addition for e.g. pH-control just as the agitation speed and gassing rate do. Standardized bioreactor formats, in terms of height-to-diameter ratio for example, can contribute to a proper scale-up.The simple way—not always the best one
Due to their low cost and easy handling, traditional shake flasks and microtiter plates are still widely used in early bioprocess development. However, these tools are poorly instrumented and offer very limited monitoring and control possibilities. Moreover, the hydrodynamics of shaken cultures completely differ from stirred-tank cultures in larger scale bioreactors. Such early-stage cultivation systems are not representative of stirred-tank bioreactor environments, and are therefore not suitable for the development of industrial production processes.A reliable scale-up strategy is better to count on miniaturized stirred-tank bioreactors, which are comparable with large-scale production reactors in terms of design and fluid dynamics.
Parameters to rely on—conventional scale-up criteria
Engineering parameters such as the vessel and impeller geometry, tip speed, mixing time, oxygen transfer rate, volumetric mass transfer coefficient (kLa), or power number can be used as scale-up criteria [3]. The general strategy is to keep a specific process parameter constant throughout the scale-up process. In this way, negative effects caused by changing environmental conditions during scale-up are minimized. Choosing the best scale-up criterion to use depends on the process.For cultures that are sensitive to shear force damage, like animal or stem cell cultures, a defined tip speed may be reasonable. On the other hand, such a strategy may limit the working volume because a constant tip speed is achieved by reducing the agitation speed with increasing impeller diameter, thus causing poorer mixing performance in larger vessels.
Lire moins
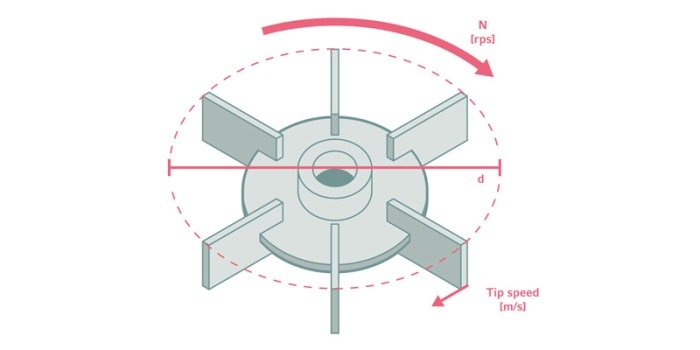
Impeller rotation vs. tip speed. d: impeller diameter
Another strategy is to match the mixing times between larger and smaller bioreactors to ensure a proper and uniform supply of nutrients, gasses, and heat to the culture.
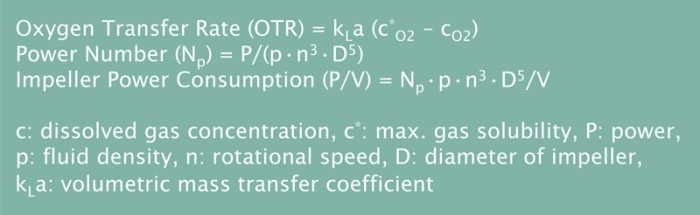
In aerobic fermentation, the transfer of oxygen is critical because oxygen is often a limiting factor for growth of the culture. In a bioprocess, the rate at which oxygen is transferred from the air to the liquid culture is called the oxygen transfer rate (OTR). For an efficient scale-up it is important to use equipment that shares similar OTR capabilities at all scales. Oxygen transfer can be also described by the kLa which can serve as scale-up criterion as well.
One of the most widely accepted scale-up strategies is to maintain a constant impeller power consumption per liquid volume (P/V). This is carried out by adapting the impeller size and shape to the different working volumes. The P/V value is calculated from the impeller power number (Np), also called Newton number (Ne), which is a dimensionless number associated with different types of impellers.
Formula:
In aerobic fermentation, the transfer of oxygen is critical because oxygen is often a limiting factor for growth of the culture. In a bioprocess, the rate at which oxygen is transferred from the air to the liquid culture is called the oxygen transfer rate (OTR). For an efficient scale-up it is important to use equipment that shares similar OTR capabilities at all scales. Oxygen transfer can be also described by the kLa which can serve as scale-up criterion as well.
One of the most widely accepted scale-up strategies is to maintain a constant impeller power consumption per liquid volume (P/V). This is carried out by adapting the impeller size and shape to the different working volumes. The P/V value is calculated from the impeller power number (Np), also called Newton number (Ne), which is a dimensionless number associated with different types of impellers.
Formula:
Lire moins
Understand complexity with multi-scale analysis
As described above, conventional scale-up is mainly based on the principle of similarity and dimensional analysis. Biological properties are usually not considered. In contrast, the multi-scale analysis deals with the complex interaction of cellular machinery and extracellular environment across all the scales. It takes a broad range of influencing and interacting factors of the complex biological cultivation system into account. Multi-scale analysis approaches require a deep understanding of the cells’ metabolism and behavior, as well as the technical capability for comprehensive measurement and incorporation of real-time analysis. With the increasing possibilities for accurate on-line monitoring and analysis of bioprocess changes and the on-line control of process parameters, the multi-scale analysis is a powerful strategy for the development of scalable and robust bioprocesses.Gradients—the large-scale drawback
Even if the design of bioreactors of different sizes is similar, identical process performance cannot be expected from one scale to another. In large-scale bioreactors, various gradients can occur due to insufficient mixing and mass transfer limitations. Dissolved oxygen, pH or substrate gradients lead to an inhomogeneous cultivation environment. The heterogeneities in large scale bioreactors may lead to multiple physiological responses in the cultivated cells and organisms. Growth and productivity can be affected which may result in lower cell and product yields or increased by-product formation, compared to lab-scale bioreactors. Therefore, an understanding of the cells’ performance in a dynamic environment is of vital importance for an efficient scale-up.
Lire moins
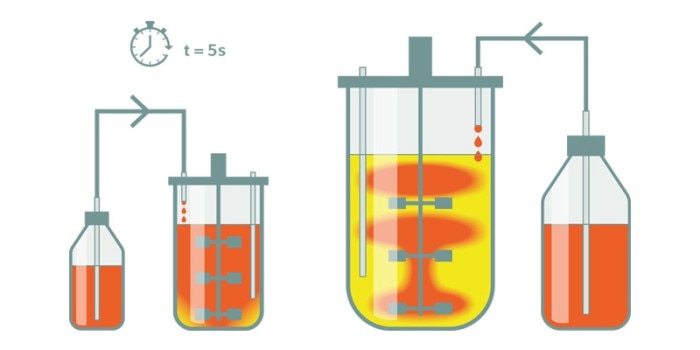
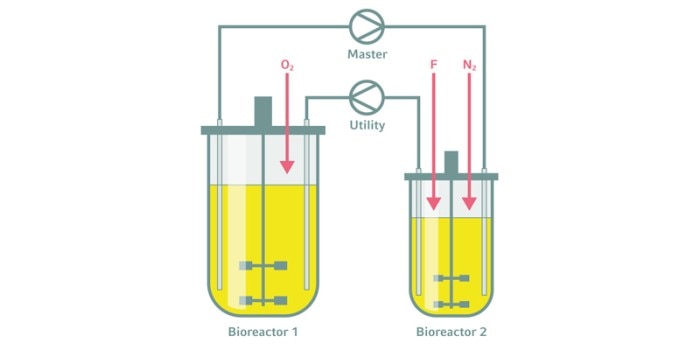
Scale-down approaches
Two-compartment bioreactors are a smart tool to mimic the heterogeneities in industrial-scale bioreactors [4]. This kind of scale-down system allows us to predict potential quality loss during scale-up, even at a laboratory-scale, process-development stage. The idea is to combine two individual bioreactors into a two-compartment bioreactor system. Each of the single bioreactors has a specific cultivation environment with its own parameter settings, representing the upper and the lower gradient environments. During cultivation, the cells are continuously transferred from one compartment to the other.The two-compartment cultivation results can give insight into bioprocess phenomena that—with conventional approaches—may have first showed up in the final industrial production process.
Lire moins
Calculating process dynamics
Beside the Newton number described above, several other dimensionless numbers can be used as parameters for mathematical modeling to describe the dynamics of a bioreactor culture. The Reynolds number (Re), for example, provides information on the culture’s turbulence. The Schmidt number (Sc) describes the mass transfer, and the Sherwood number (Sh) reflects the effective mass transfer. Computational fluid dynamics (CFD) is a model-based method to simulate culture dynamics at different scales. It can be a useful tool for predicting and solving scale-up problems including mixing, mass transfer or fluid flow.Say it by numbers—mathematical simulation systems
The rapid development of computer hardware and simulation algorithms creates very new opportunities. Complex mathematical models can be used to understand, predict, and optimize the properties and behavior of cultured cells and indeed the whole bioprocess. Such integrated models are coupling fluid dynamics and cell kinetics. They can be used to simulate hydrodynamic effects and physiological responses in various kinds of bioreactors and at different scales.Challenging but feasible—a conclusion
Every bioprocess is a complex system. The living cell or organism that is cultured is in continuous interaction with itself, other cells and its cultivation environment. Every change in cultivation parameters triggers the cells’ internal control mechanisms and activates responses. Moreover, with increasing bioreactor size, heterogeneities occur and the cells need to adapt to a rapidly changing environment.Using scale-up strategies that consider the challenges mentioned above, in combination with truly scalable bioreactor equipment can offer efficient approaches to scaling-up bioprocesses from early development to final production.
Lire moins
References:
[1] Marques MPC, Cabral JMS and Fernandes P. Bioprocess scale-up: quest for the parameters to be used as criterion to move from microreactors to lab-scale. Journal of Chemical Technology and Biotechnology 2010; 85(9): 1184-1198
[2] Xia J, Wang G, Lin J, Wang Y, Chu J, Zhuang Y and Zhang S. Advances and Practices of Bioprocess Scale-up. Adv Biochem Eng Biotechnol 2016; 152:137-51
[3] Li B, Becken U and Sha M. Tackling the Challenge of Scalability. GEN 2016; 36(9); https://www.eppendorf.com/product-media/doc/en/163815/DASGIP_Fermentors-Bioreactors_Publication_DASGIP-Parallel-Bioreactor-System_BioFlo-320_610_Pro_Tackling-challenge-scalability.pdf
[1] Marques MPC, Cabral JMS and Fernandes P. Bioprocess scale-up: quest for the parameters to be used as criterion to move from microreactors to lab-scale. Journal of Chemical Technology and Biotechnology 2010; 85(9): 1184-1198
[2] Xia J, Wang G, Lin J, Wang Y, Chu J, Zhuang Y and Zhang S. Advances and Practices of Bioprocess Scale-up. Adv Biochem Eng Biotechnol 2016; 152:137-51
[3] Li B, Becken U and Sha M. Tackling the Challenge of Scalability. GEN 2016; 36(9); https://www.eppendorf.com/product-media/doc/en/163815/DASGIP_Fermentors-Bioreactors_Publication_DASGIP-Parallel-Bioreactor-System_BioFlo-320_610_Pro_Tackling-challenge-scalability.pdf
Lire moins